Abstract
Background: NEL is effective for patients (pts) with relapsed T-ALL/LBL, but its incorporation into the front-line therapy has not been investigated extensively in the adult population. In children, the addition of NEL to the induction regimen has shown to improve disease-free survival (DFS) but not the overall survival (OS). VEN has shown preclinical and clinical activity in relapsed T-ALL/LBL. We investigated the addition of NEL and PEG to the hyper-CVAD (HCVAD) regimen in adult pts with T-ALL/LBL. Further on, we explored the safety and efficacy of adding VEN to this regimen.
Methods: Pts with previously untreated or minimally pre-treated T-ALL/LBL were eligible if they had an ECOG PS ≤3, creatinine ≤ 2 mg/dL, total bilirubin ≤ 2 mg/dL and ALT/AST ≤ 4 x ULN. Pts received 8 cycles of HCVAD (cycles 1,3, 5, 7) alternating with high dose ara-C and methotrexate (MTX) (cycles 2, 4, 6, 8) at approximately 3-week intervals. Two cycles of NEL (650 mg/m2 daily x 5) were initially administered after cycle 8 (cohort 1). Later, after a protocol amendment, they were administered after cycle 4 and cycle 5 (cohort 2). Subsequently, the protocol was amended to add PEG (1500 IU/m2 capped at 3750 IU) on day 5 of the NEL cycles (cohort 3) and more recently, VEN 400 mg daily was added on the first 7 days of each of 8th cycle of therapy (cohort 4) and this was later modified to be given for 7 days during the induction cycle and reduced to 3 days per cycle post-induction only in pts with ETP-ALL or those with persistent MRD. All other pts did not receive VEN after cycle 1 (cohort 5). Pts with ETP-ALL were referred for allogeneic stem cell transplant in first complete remission (CR). After the completion of the intensive phase, pts in all cohorts received 30 cycles of maintenance therapy with monthly POMP and early intensification with NEL/PEG on cycles 6 and 7 and late intensification with MTX/PEG in cycle 18 and HCVAD on cycle 19. All pts received 8 intrathecal chemotherapy with MTX alternating with ara-C and mediastinal radiation was considered in pts with bulky mediastinal disease.
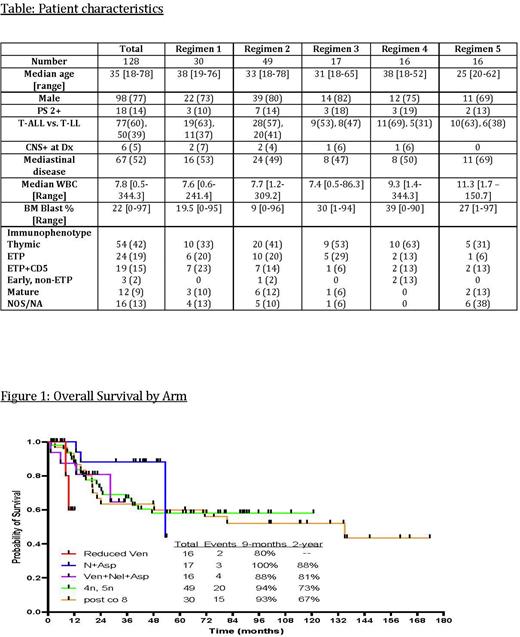
Results: Between 8/2007 and 6/2022, 128 pts were enrolled in the 5 study cohorts sequentially (cohort 1= 30, 2= 49, 3=17, 4=16, 5=16) (Table). Seventy-seven pts had T-ALL, 50 T-LBL and 1 biphenotypic T/myeloid leukemia. Median age was 35 yrs. (range 18-78), 98 pts were male (77%) and 110 pts (86%) had PS 0-1. In pts with T-ALL, median bone marrow blast percentage was 83% (range, 20-97) Six pts (5%) had CNS disease at presentation. Cytogenetics were diploid in 82 pts (64%), abnormal in 36 (28%), and unavailable in 10 pts (8%). Pts were further characterized as early T-cell precursor (ETP; n=24), near ETP (ETP + CD5) (N=19), early-non-ETP (n=3), thymic (n=54), mature (n=12), not otherwise specified (NOS; n=16). Twenty-five pts had received 1-2 cycles of therapy prior to enrollment, with 18 having achieved CR. The 30-day mortality was 0%. One hundred and twelve pts (88%) had ≥ 1 grade 3/4 non-hematological treatment-emergent adverse events (TEAE) with the most frequent grade 3/4 TEAEs being neutropenic infections in 96 pts (75%) and elevated liver enzymes in 25 pts (20%). The overall response rate (CR/CRi/PR) was 98% with CR/CRp in 120/128 pts (94%), PR in 5 (4%), and no response in 3 (2%). The median number of cycles to response (including a negative PET scans) was 1 (range 1-10). CR/CRp rate in T-ALL was 91% and T-LBL was 98%. At a median follow-up of 50 months (1-174), 84 patients (66%) are alive with a 5-year PFS and OS of 60% and 61% (Figure 1) and 79 pts (62%) remain in CR.
Conclusion: The addition of NEL/PEG cycles to the HCVAD regimen is feasible. The addition of VEN to each cycle is associated with significant myelosuppression.
Disclosures
Kantarjian:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Jabbour:AbbVie: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Konopleva:Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Ablynx: Other: Grant support, Research Funding; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Kisoji: Consultancy, Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Amgen: Consultancy; Forty-Seven: Consultancy, Honoraria, Other: Grant support; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Novartis: Patents & Royalties, Research Funding; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; Genentech: Consultancy, Other: grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding. Wierda:Miragen: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; AbbVie: Research Funding; Sanofi: Consultancy; Sunesis: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Kite, a Gilead Company: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Cyclacel: Research Funding; Pharmacyclics LLC: Research Funding; Xencor: Research Funding; Genzyme: Consultancy; Genentech: Research Funding; Gilead Sciences: Research Funding; GSK/Novartis: Research Funding; Janssen: Research Funding; Juno: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; Karyopharm: Research Funding. Burger:Janssen: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy; Gilead: Consultancy; BeiGene: Consultancy, Research Funding; Pharmacyclics LLC: Consultancy, Research Funding; Novartis: Consultancy. Issa:Celgene, Kura Oncology, Syndax, Merck, Novartis: Research Funding; Novartis, Kura Oncology: Consultancy. Ferrajoli:Beigene: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Aprea: Honoraria; Acceleron Pharma: Consultancy; Curis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Alvarado:BerGenBio: Research Funding; Astex Pharmaceuticals: Research Funding; FibroGen: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Research Funding. Short:AstraZeneca: Consultancy; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Astellas: Research Funding; Novartis: Consultancy; Takeda Oncology: Consultancy, Research Funding; Stemline Therapeutics: Research Funding. Jain:Ipsen: Honoraria; Aprea Therapeutics: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; MEI Pharma: Honoraria; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte Corporation: Research Funding; ADC Therapeutics: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; Loxo Oncology: Research Funding; Fate Therapeutics: Research Funding; Mingsight: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Novalgen: Research Funding; Newave: Research Funding; Pfizer: Research Funding; Cellectis: Honoraria, Research Funding; CareDx: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Servier Pharmaceuticals LLC: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Beigene: Honoraria; TransThera Sciences: Research Funding; Takeda: Research Funding; Medisix: Research Funding; Dialectic Therapeutics: Research Funding. Ravandi:Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding; AstraZeneca: Consultancy; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.